A harpacticoid specimen was obtained subtidally at Oshoro Bay, Hokkaido, Japan, about 43°12′N, 140°51′E, on 7 June 2010 by Miku Kato, photographed by Hiroshi Kajihara and fixed in 99% EtOH. DNA was extracted from the entire body, using the silica method (Boom et al. 1990) with some modifications. Extracted DNA was dissolved in 30 µl of deionized water and has been preserved at –20°C.
Amplification of mitochondrial cytochrome c oxidase subunit I gene (COI) using LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. 1994) was unsuccessful.
An about 1.2 K-bp fragment of mitochondrial 28S rRNA gene was amplified by polymerase chain reaction (PCR) using LSU5 (5′-ACCCGCTGAAYTTAAGCA-3′) and LSU3 (5′-TCCTGAGGGAAACTTCGG-3′) (Littlewood DT. 1994). A hot start PCR was performed by a thermal cycler, iCycler (Bio-Rad), in a 20-µl reaction volume containing 1 µl of template total DNA (approximately 10–100 ng) and 19 µl of premix made with 632-µl deionized water, 80-µl Ex Taq Buffer (TaKara Bio), 64-µl dNTP (each 25 mM), 8-µl each primer (each 10 µM), and 0.1-µl TaKara Ex Taq (5 U/µl,TaKara Bio). Thermal cycling condition comprised an initial denaturation at 95°C for 30 sec; 30 cycles of denaturation at 95°C for 30 sec, annealing at 45°C for 30 sec, and elongation at 72°C for 45°C and a final elongation at 72°C for 7 min.
The PCR product was purified with the silica method (Boom et al. 1990). Both strands were sequenced with a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) following the manufacturer's protocol, using the same primer set as the initial PCR amplification. Sequencing was performed with ABI Prism 3730 DNA Analyzer (Applied Biosystems). Chromatogram and sequence data were operated with MEGA v4 software (Tamura et al. 2007).
Results
A total of 649 bp of 28S rDNA sequence was determined with certainty from Harpacticoida sp. (see Appendix).
Taxonomy
Subphylum Crustacea
Subclass Copepoda
Order Harpacticoida
Family uncertain
(Figs 1, 2)
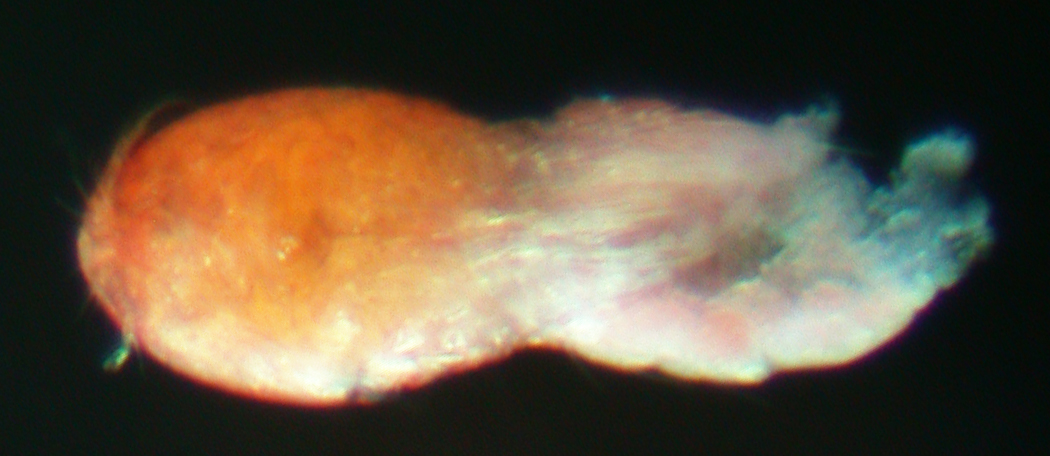
Fig. 1. Harpacticoida sp. (ICHU22080229), entire view.
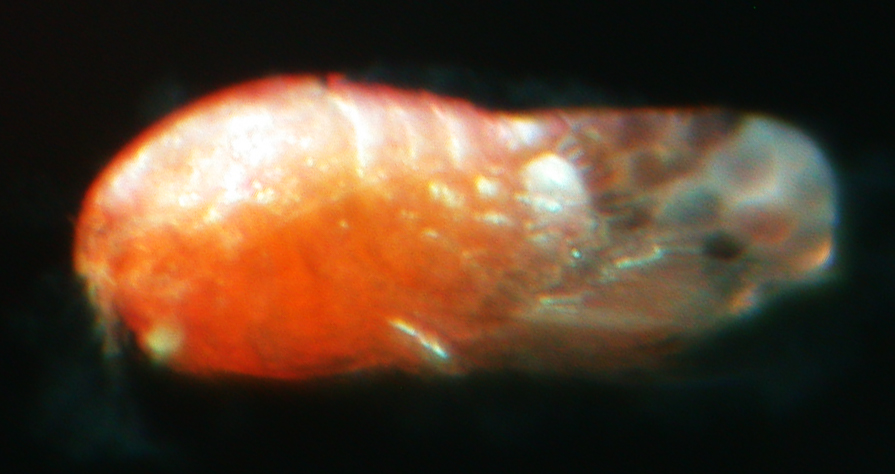
Fig. 2. Harpacticoida sp. (ICHU22080229), entire view.
References
Boom, R., Sol., C. J. A., Salimans, M. M. M., Jansen, C. L., Wertheim-van Dillen, P. M. E., and van der Noordaa, J. 1990. Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology28: 495–503.
Folmer, O., Black, M., Hoeh, W., Lutz, R. and Vrijenhoek, R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Littlewood, D. T. 1994. Molecular phylogenetics ofcupped oysters based on partial 28S rRNA gene sequences. Molecular Phylogenetics and Evolution 3: 221–229.
Tamura, K.,Dudley, J., Nei, M. and Kumar, S. 2007. MEGA4: Molecullar Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Phylogenetics and Evolution 24: 1596–1599.
Appendix
28S rRNA gene sequence from Harpacticoida sp. (ICHU22080229).
CGATTAGTCTTTCGCCCCTATACCCAGCTCCGACGATCGATTTGCACGTCAGAATCGCTACGGTCCTCCATCAGGGTTTCCCCTGACTTCAACCTGGCCAGGCATAGTTCACCATCTTTCGGGTCCCAACGTGTATGCTCTTGCTGCTCCGCACGATGCGAGGTCGATGCGAAGCGCCGGGATTGCGCTCGTGAGAGATCATCCCTAAGCTACTGAGTAGCCTTTGCTTTCACTACGCCTTTAGGTTTAGT-CAACCCAATGACTCGCACACATGTTAGACTCCTTGGTCCGTGTTTCAAGACGGGTC---AGAGGCACCGCCGACCTACTCGCAACTGAGATGCAAGCCGTGCCAGTGAAGACACTC-GCTATACGAGCCTGCTTAGCTACCCGCCGGGCCCGGAGCTAG-GAACGCGGGGCTTGGGACAAGTCCCAAACTTCACCCACGGCCTCAGTC-CAGTCCCAGC--GGTTCGTTGCGTGAAGAGCCAAAGAAATGCTGCCACCGAGGACACTCACCACCGCACCGAGTCCTTGCGGATCCCGAG--AACAGCAGCAGAGCCCCCGTTAGCATGAACCTCCAGCTCCGACTTCGAGAATGCCTCTGTTTACCACTACACAGTTTCACGTACTCTTGAACTCTC