A polychaete was obtained among laminarian holdfast at Oshoro Bay, Hokkaido, Japan, about 43°12′N, 140°51′E, on 13 June 2011 by Ayako Tanaka, photographed by Hiroshi Kajihara, then fixed in 99% EtOH. The specimen was later identified as Amphicorina mobilis based on Yoshihara et al. (2012). DNA was extracted from the whole specimen, using the silica method (Boom et al. 1990) with some modifications. Extracted DNA was dissolved in 30 μl of deionized water and has been preserved at –20°C.
Amplification of mitochondrial cytochrome c oxidase subunit I gene (COI) using LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. 1994) was unsuccessful.
An about 1.2K-bp fragment of 28S rRNA gene was amplified by polymerase chain reaction (PCR) using LSU5 (ACCCGCTGAAYTTAAGCA) and LSU3 (TCCTGAGGGAAACTTCGG) (Littlewood. 1994). A hot start PCR was performed by a thermal cycler, iCycler (Bio-Rad), in a 20-μl reaction volume containing 1 μl of template total DNA (approximately 10–100 ng) and 19 &mul; of premix made with 632-μl deionized water, 80-μl Ex Taq Buffer (TaKara Bio), 64-μl dNTP (each 25 mM), 8-μl each primer (each 10 μM), and 0.1-μl TaKara Ex Taq (5 U/μl,TaKara Bio). Thermal cycling condition comprised an initial denaturation at 95°C for 30 sec; 30 cycles of denaturation at 95°C for 30 sec, annealing at 45°C for 30 sec, and elongation at 72°C for 45°C and a final elongation at 72°C for 7 min.
The PCR product was purified with the silica method (Boom et al. 1990). Both strands were sequenced with a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) following the manufacturer's protocol, using the same primer set as the initial PCR amplification. Sequencing was performed with ABI Prism 3730 DNA Analyzer (Applied Biosystems). Chromatogram and sequence data were operated with MEGA version 5.0 (Tamura et al. 2011).
Results
Two regions of 28S rDNA sequence were determined from Amphicorina mobilis (Rouse, 1990) (see Appendix).
Taxonomy
Phylum Annelida
Class Polychaeta
Order Aciculata
Family Sabellaridae
Genus Amphicorina Claparède, 1864
Amphicorina mobilis (Rouse, 1990)
(Fig. 1)
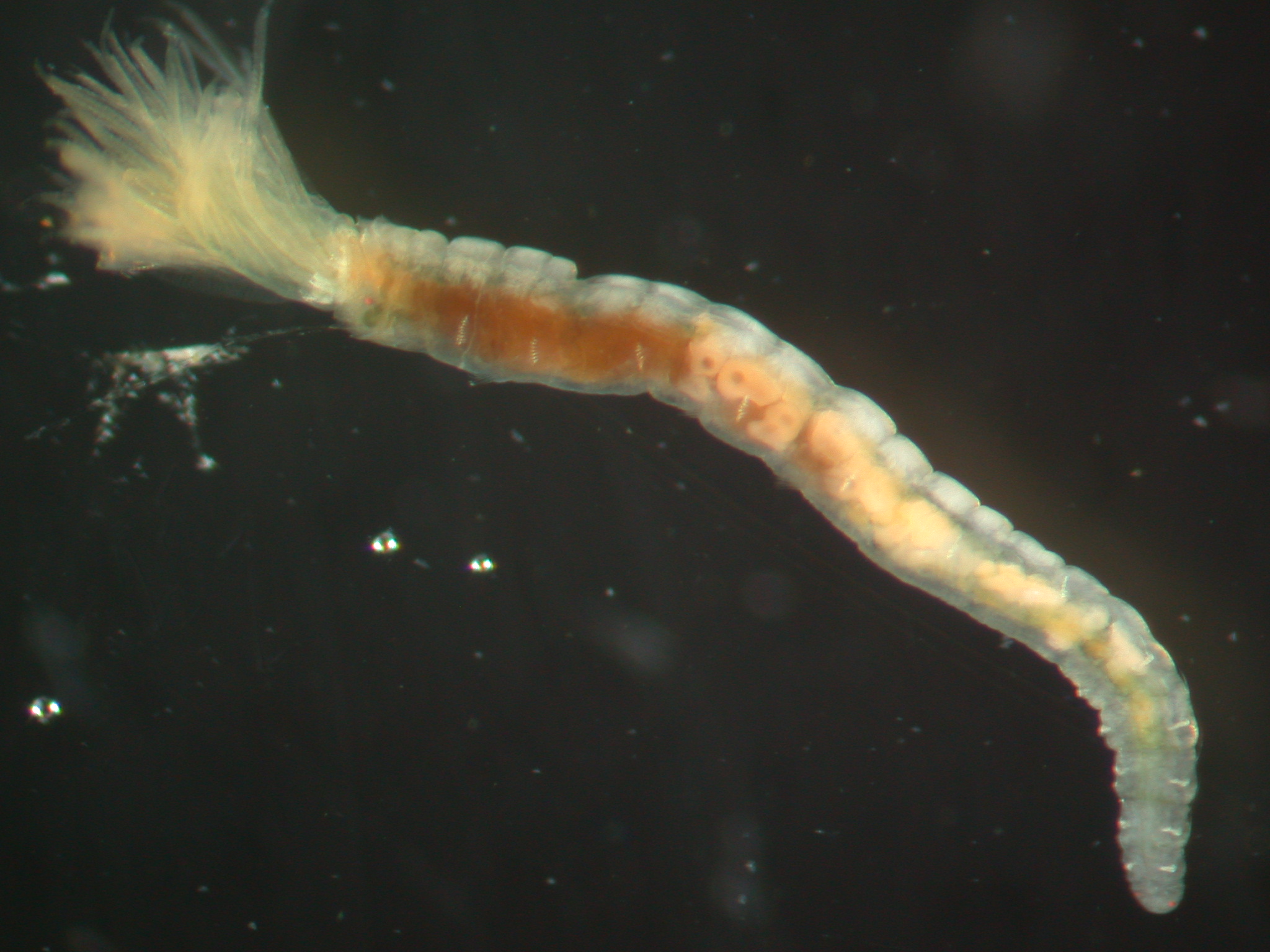
Fig. 1. Amphicorina mobilis (Rouse, 1990) (ICHU22090290), overall view.
References
Boom, R., Sol, C. J. A., Salimans, M. M. M., Jansen, C. L., Wertheim-van Dillen, P. M. E., and van der Noordaa, J. 1990. Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology 28: 495–503.
Folmer, O., Black, M., Hoeh, W., Lutz, R. and Vrijenhoek, R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Littlewood, D. T. 1994. Molecular phylogenetics ofcupped oysters based on partial 28S rRNA gene sequences. Molecular Phylogenetics and Evolution 3: 221–229.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. and Kumar, S. 2011. MEGA5: molecullar evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Phylogenetics and Evolution 28: 2731–2739.
Yoshihara, T., Hiruta, S., Katoh, T. and Kajihara, H. 2012. Three species of Amphicorina (Annelida, Sabellida, Sabellidae) from Japan, with descriptions of two new species. ZooKeys 187: 45–62.
Appendix
28S rDNA sequence from ICHU22090290 identidied as Amphicorina mobilis (Rouse, 1990).
GGCGCTGGGGAACTGTGGTGTTTGGGAGAGCCTCTGGTGCGTGCCGCCTGCCGTCCGAGTCCTCCTGATTGGGGCTTCGCCCGTAGCGGGTGTCAGGCCTCTACGGCGGCGGCGGCCGTGCCTCTGAGCCTCCCCGGAGTCGGGTTGTTTGAGAATGCAGCCCAAAGTGGGTGGTAAACTCCATCTAAGGCTAAATACCGACACGAGACCGATAGCGGACAAGTACCGTGAGGGAAAGTTGAAAAGAACTTTGAAGAGAGAGTTCAAGAGTACGTGAAACCGTTTAGAGGCAAACGGATAGGACCGCAAAGTCCGCCCGCGGAATTCAACCTCGCCGGACCCGTCCTGCGCGGTGTCTTCGGATCGTCCGTACGGGCGAACGGGGGTCTGCGTCTTGGGCGGGCCGAGGGGCGCACTTTCCGCGGGGGGAGCGCCACGACCGGTTCCTGGGCCGTCATGAGCCCCGGCGGAAGGTAGCCGGACCCTTCGGGGTTTGGTGTTACAGACGTCGGTGGTGGGCCGGCTCGGGGGACCGAGGATAATACCCGCCGCGCGAGGTGGGCGTCGGGGGGCTGTCGCCGGTTTCGACTG
GAAGGGTGCGACGCGGTCGCCACAGACCCTGTGTGCGCGGGCGGAGCAGCCAGGGAAGAAGCCGCCAGCGGTCGAACGGCACTGCGAGCAGTCCCGCCCAGTCGAAACCGGCGACAGCCCCCCGACGCCCACCTCGCGCGGCGGGTATTATCCTCGGTCCCCCGAGCCGGCCCACCACCGACGTCTGTAACACCAAACCCCGAAGGGTCCGGCTACCTTCCGCCGGGGCTCATGACGGCCCAGGAACCGGTCGTGGCGCTCCCCCCGCGGAAAGTGCGCCCCTCGGCCCGCCCAAGACGCAGACCCCCGTTCGCCCGTACGGACGATCCGAAGGCACCGCGCAGGACGGGTCCGGCGAGGTTGAATTCCGCGGGCGGACTTTGCGGTCCTATCCGTTTGCCTCTAAACGGTTTCACGTACTCTTGAACTCTCTCTTCAAAGTTCTTTTCAACTTTCCCTCACGGTACTTGTCCGCTATCGGTCTCGTGTCGGTATTTAGCCTTAGATGGAGTTTACCACCCACTTTGGGCTGCATTCTCAAACAACCCGACTCCGGGGAGGCTCAGAGGCACGGCCGCCGCCGCCGTAGAGGCCTGACACCCGCTACGGGCGAAGCCCCAATCAGGAGGACTCGGACGGCAGGCGGCACGCACCAGAGGCTCTCCCAAACACCACAGTTCCCCAGCGCCGCAAGGCACGGGGGATTCGGTGCTGGGCTCTTCCCTCTTCACTCGCCGTTACTGTGGGAATCCTTGTTAGTTCTTTTCCCTCCGCTTAGTGATATGCTTAAATTCGGGGGGGGTAGTCC