A Japanese oyster was obtained from a tide pool in Oshoro Bay, Hokkaido, Japan, about 43°21′N, 140°85′E, on 26 May 2014 by Akane Asano and Nozomi Oshima, identified by Hiroshi Kajihara as Crassostrea gigas (Thunberg, 1793), then photographed and fixed in 99% EtOH by Takumi Onishi. DNA was extracted from the muscle tissue using the silica method (Boom et al. 1990) with some modifications. Extracted DNA was dissolved in 30 µl of deionized water and has been preserved at –20°C. Remaining morphological voucher specimen has been deposited at the Hokkaido University Museum under the catalogue number ICHU2210066 (contact: Dr. Hiroshi Kajihara, kazi@mail.sci.hokudai.ac.jp).
An about 650-bp fragment of the mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified by polymerase chain reaction (PCR) using the primer pair LCO1490 (5′- GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA -3′) (Folmer et al. 1994). A hot start PCR was performed by a thermal cycler, 2720 Thermal Cycler (Applied Biosystems), in a 20-µl reaction volume containing 1 µl of template total DNA (approximately 10–100 ng) and 19 µl of premix made with 632-µl deionized water, 80-µl Ex Taq Buffer (TaKara Bio), 64-µl dNTP (each 25 mM), 8-µl each primer (each 10 µM), and 0.1-µl TaKara Ex Taq (5 U/µl,TaKara Bio). Thermal cycling condition comprised an initial denaturation at 95°C for 30 sec; 30 cycles of denaturation at 95°C for 30 sec, annealing at 45°C for 30 sec, and elongation at 72°C for 45 sec; and a final elongation at 72°C for 7 min.
The PCR product was purified with the silica method (Boom et al. 1990). Both strands were sequenced with a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) following the manufacturer's protocol, using the same primer set and new primer set, D2F (5′-CTTTGAAGAGAGAGTTC-3′) (Littlewood et al. 1994) and 28z (5′-CTTGGTCCGTGTTTCAAGAC-3′) (Hillis and Dixon et al. 1991) as the initial PCR amplification. Sequencing was performed with ABI Prism 3730 DNA Analyzer (Applied Biosystems). Chromatogram and sequence data were operated with MEGA v.5 software (Tamura et al. 2011).
Results
Phylum Mollusca
Class Bivalvia
Family Ostreidae Rafinesque, 1815
Genus Crassostrea Sacco, 1897
Crassostrea gigas (Thunberg,1793)
(Fig. 1)
Due to the poor chromatogram data, no reliable sequence was obtained.
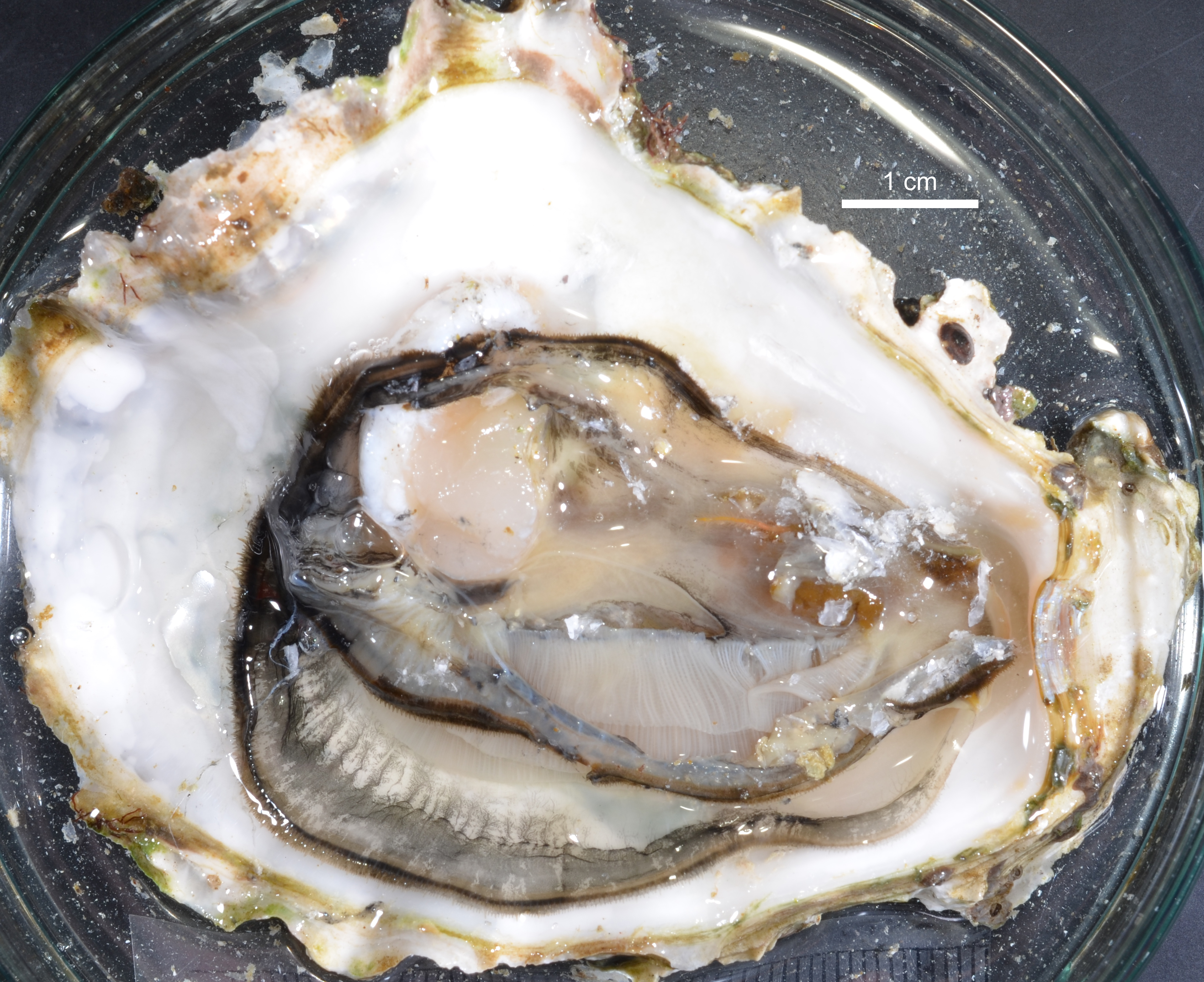
Fig. 1. Crassostrea gigas (Thunberg, 1793), ICHU2122045, photograph taken in life.
References
Boom, R., Sol, C. J. A., Salimans, M. M. M., Jansen, C. L., Wertheim-van Dillen, P. M. E., and van der Noordaa, J. 1990. Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology 28: 495–503.
Littlewood, D. T. 1994. Molecular phylogenetics of cupped oysters based on partial 28s rRNA gene sequences. Molecular Phylogenetics and Evolution 3 221–229.
Hills, D. M. and Dixon, M.T. 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Quarterly Review of Biology 66 441–453
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. 2011. MEGA5: Molecullar Evolutionary Genetics Analysis (MEGA) software version 5.0. Molecular Phylogenetics and Evolution 28: 2731–2739.